"The subject matter of the patents and the prior art is of great technical complexity, and there are lengthy textbooks concerning genetic engineering and immunology, which are the relevant areas of expertise."
And complex it indeed is, though the judge doesn't seem to struggle half as much in writing about the invention as your correspondent did in reading about it. In case it's not clear, this is entirely an admission of this Kat's technical limitations and not in any way to be read as a criticism of Mr Justice Carr's powers of explanation; anyone reading the judgment will be impressed with how he got to grips with this complex technology. As a typical example, the judge nonchalantly explains at one point that:
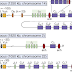
 |
| See? A picture makes everything clear. |
“The preamble to the claim identifies the product to be produced by the method, namely a heavy chain variable region gene locus which has been modified in an ES cell so that V, D, and J gene segments of the endogenous locus have been replaced with orthologous human V, D and J gene segments, to create a modified immunoglobulin locus that produces hybrid antibodies containing human variable regions and mouse constant regions (i.e. a reverse chimeric locus).”
And that’s just what the preamble to the claim is about. The complicated stuff is in the claim body.
Therefore, what follows in this post may be a grotesque oversimplification of a lengthy and complex patent decision. If you’re practising in this field and want to know more, the IPKat suggests you quench your thirst from the source, which is Regeneron Pharmaceuticals Inc v Kymab Ltd & Anor [2016] EWHC 87 (Pat).
The invention relates to modifying a mouse genome by swapping out specific segments and replacing them with a human gene segments. The resulting sequence can be used to prepare antibodies with therapeutic uses. There's much more to it as you might suspect, but the issues ultimately revolved around the deletion of native mouse genes and their replacement in situ by human genes.
There were two patents at issue, a parent and a divisional, both of which were held to be infringed. Validity attacks alleging lack of novelty and inventive step failed, as did an objection of added subject-matter. However, the defendants succeeded in having the patents revoked for insufficiency.
Applying the usual principles that the whole subject-matter defined in the claims must be capable of being performed without undue burden and without invention, the judge construed the claims to decide what was properly covered by the claims. The critical issue related to the length of the sequences swapped in and out of the mouse genome.
At the priority date (2001), the skilled person would have had the ability to successfully swap in and out sequences with lengths of the order of 4 kb (i.e. 4,000 base pairs) to about 20 kb. The patentee’s trials in 2002 showed an average deletion of 8 kb and an average replacement of 4.5 kb. Even allowing for the fact that this was a technical field where a great deal of trial and error was often called for, this fell far short of what would have been required to put the invention into practice across the claim's breadth.
The judge held that the claims covered the deletion of mouse sequences of 100 kb and upwards, and the insertion of human sequences of 75 kb to 300 kb. The expert evidence showed that manipulations of this size were far beyond the capabilities of the skilled person at the priority date and, at the upper limits of the claim, were perhaps even beyond current capabilities:
217. I have concluded that:
i) the minimum replacement by LTVEC1 that is described in [0125] is a deletion of 100 kb of mouse sequence [...] and an insertion of 200-300 kb of human sequence (as shown in Fig.4);ii) the minimum replacement that is required by claim 1 of the 287 patent involves a larger deletion (150 kb) but a smaller insertion (75 kb); andiii) The claim also includes the case where the mouse sequence is displaced and deactivated, but it is not limited to exclude deletion of the mouse sequence, which is specifically described in the specification. [This last point had been an issue of claim construction: what did “in situ” replacement mean?]
218. In my judgment, it was clear from the evidence of Profs. Evans and Stewart that insertions and deletions of this size could not be performed without undue burden in 2001/2, and that the likelihood was that neither of the insertions and deletions referred to in (i)-(ii) above would have worked.
219. In particular, Prof. Evans agreed that insertion of 75 kb by homologous recombination was considerably larger than what had been achieved by 2001/2. As to deletion, he agreed that there is no publication even now of a deletion by homologous recombination of 100 kb or more of the mouse genome. It follows that a replacement of 200-300 kb of human sequence for 100 kb of mouse (or of 75 kb human for 150 kb mouse) was considerably larger than any that had been achieved by homologous recombination at the priority date.These conclusions applied to all the independent claims at issue in both patents (some of which were broader than claim 1 of the ‘287 patent discussed in this passage). As a result, the patents were revoked.
This Kat did not manage to spot anything unorthodox in how the judge approached sufficiency, or in his application of the tests as developed over the years both by the courts in the United Kingdom and by the EPO Boards of Appeal.
As always, however, the IPKat would like to hear what readers think, particularly those practising in the life sciences, or at least the subset which has not succumbed to apoplexy upon seeing their beloved subject tortured beyond recognition when described by someone who took the electrical/mechanical exam option whenever he could possibly do so.
 Reviewed by David Brophy
on
Friday, February 19, 2016
Rating:
Reviewed by David Brophy
on
Friday, February 19, 2016
Rating:




![[GuestPost] G1/24: Tuning in! A take on the state of proceedings before oral proceedings](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjZhEivE5bp7QOwZsyZXAXbVNYSmLjUthkB2Q7fm1_dpB97u5lIQeyWT9ZadUTAH3Z-hXn13VpW4vBDRPx9emCnoDV6tbUTkyvfmqPv1nNInL8XMdrAtSZ2hcRQr2LjxKovC9wTk_XyZxQ0CtX1MUrO_Muz3OJ4ld8AftymsdUmKD7xNksYMwk6/s150/Picture%201.png)
![[Guest post] ‘Ghiblification’ and the Moral Wrongs of U.S. Copyright Law](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhxl1BQBAW3Y-asjb1xXB9eA4DYy77fky6WgR-prC-_6DeBbDqOgCUDWyiz0Q3B23MWWAXnkbS2H2js7OUwA0JQXAHmsyVFgGIHeJz7zJ791vTzOD-4SJqWFIuywFXQyd3ahybbdZd4e8IEVfcNqctvyR8lumv_Gix6Tsw5cSvbHpTI1nwvztDuAQ/s150/IMG_2179.HEIC)


![[Guest book review] The Handbook of Fashion Law (with a discount code)](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgB4h2AdqJKwq9O3Ft4Mb7C39tv_NeFpkzrOfvhIsuwAkM_ops2Hgj7fdwzq_TQsjQDvQrQa-yyC9Q9pNiugseXRlUaMdsr_cmYUbh9lH8HDECMCbsTuNboVgpafyEhkgDkVS6ruHkuz8Sx0QVGI_1S8R9kbsHdNIYrRjqhyphenhyphen010_txjJUYvlZOtWA/s150/Fashion%20Law%20Book%20Bicture.jpg)







A well reasoned and IMO absolutely correct decision.
ReplyDeleteSince Merck v Ono, I wasn't sure if "the whole subject-matter defined in the claims must be capable of being performed without undue burden and without invention" really was the usual principle any more. It's comforting to see that some judges still think it is.