 elsewhere here, here or here) that their anti-cancer drug Glivec (imatinib) should be patentable in India.
elsewhere here, here or here) that their anti-cancer drug Glivec (imatinib) should be patentable in India.Right: Imatinib mesilate
To comply with TRIPs requirements, India modified its patent law in 2005, allowing new chemical entities (post 1995) to be patented. The law was, however, also modified to prevent the practice of 'evergreening', making small modifications to a chemical entity to acquire a new patentable drug, thus extending the patented life of the drug. The amendment to the Indian Patents Act, at section 3, reads:
"The following are not inventions within the meaning of this Act, -
[...]
(d) the mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant".
The explanation provided in the amendment goes:
"For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance, unless they differ significantly in properties with regard to efficacy."
This clause therefore effectively prevents drugs that are not new chemical entities from being patentable. Patenting new salts or crystal forms is not possible, unless there is some improved efficacy.
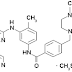
The Indian government's argument is that Glivec is not a new entity because forms of it were known of before 1995, and Glivec does not show any enhancement in efficacy. Novartis, however, argue that Glivec is more easily absorbed by the body and is therefore more effective. More significantly, Novartis also argue that the law as amended is unconstitutional, and does not comply with India's requirements under TRIPs.
The case has prompted a public outcry, including demonstrations in New Delhi. Many people view Novartis' appeal as being an attempt to restrict the availability of medicines to people who may not be able to afford them, because generic manufacturers will not be able to cheaply produce copies of patented drugs to supply the large market in India and elsewhere.
The IPKat is on the fence on this one. If Glivec is really no better in efficacy than previously available drugs, then it shouldn't really matter whether Novartis gets a patent for it or not. Generic suppliers can simply make and sell the old drug, which will work just as well. On the other hand, if Glivec really is substantially better than what was available before, don't Novartis deserve to get a patent for it, in accordance with Indian patent law? Fundamentally, it seems to come down to the old argument of whether new and innovative drugs should be the subject of patent protection at all.
 Reviewed by David Pearce
on
Monday, January 29, 2007
Rating:
Reviewed by David Pearce
on
Monday, January 29, 2007
Rating:



![[Guest post] Can AI be considered a PHOSITA? Policy debates in the US and the EU](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEggxDO6mW5r4n3-06Af5ckmIaMIhzgPJBoDP8AUsSYXY2zajUQt1ObGVn_GhCgidbG_YDVnybQuJ5XoAjjBG9Ws2xJWDQHNPMrLkn526SWIG371X_Kjt1E8tJmn8Ae_20Phyphenhyphen09JeuUOhhTR3aZE9lMRQlqHGQGcAWGxlo91rrKcLk0AfUjWCvw6RQ/s72-c/Two-cats-eating.jpg)












Re: argument in last paragraph; if Glivec is indeed better (or even different) in terms of efficacy, then it certainly deserves a grant; however Novatis are claiming the advancement is in how well the drug is absorbed. As far as I've read about the case, there is no scientifically sound way of showing this apart from doing a comparative trial. This is highly time-consuming, prone to a large error margin and could be easily manipulated.
ReplyDeleteThe dual pronged challenge by Novartis is quite interesting. Their second line of argument i.e. section 3(d) of the Indian Patent Act is Unconstitutional & in defiance of TRIPs obligations is quite amusing. Before 2005 no chemical substances were patentable; only the method of production could be claimed. And the constitution has not been amended since; so the Patent Office's objection against Glivec couldn't possibly be unconstitutional. And even if Novartis is correct with regard to TRIPs obligations, it's only an obligation for the government & not a legal argument that could stand in court.
Raj
Moreover, if there truly is a TRIPs compliance issue, why aren't they going to the WTO TRIPs Dispute Settlement Body? It'd save them the trip to India as well...
ReplyDeleteI read this article with great interest. Under European law, for new chemical products a similar effect is achieved by the inventive step requirement (Art.56 EPC). Firstly it is determined which product constitutes the closest state of the art, by analysis of all relevant prior art to see which known chemical product, having the same use or the most closely related use, is structurally closest to the claimed one. If the structural differences between the claimed product and the known one are small, without any advantages being demonstrated for the new product, it is more likely that an inventive step (obviousness) objection will result.
ReplyDeleteIn apparent difference to the Indian law, in the European system the applicant may then supply comparative test results to demonstrate an advantage of his product over that of the closest prior art (see the decision of the EPO board of appeal T197/86, OJ EPO 1989, 371). This is possible provided that any advantage demonstrated is demonstrated for the entire claimed area (patent claims rarely relate to one individual product but are usually generalisations) and that the advantages shown are related to the effects of the product already mentioned in the application as originally filed. For example if the application as originally filed indicates that the claimed product is an antibiotic but makes no mention of how effective an antibiotic it is, the applicant may subsequently prove in examination proceedings that it is more active as an antibiotic than the products of the closest prior art, even though no such allegation was made in the application as filed (see the decision of the EPO board of appeal, T184/82, OJ EPO 1984, 261), although such advantages must be proven by appropriate tests (see the decision of the EPO board of appeal T20/81, OJ EPO 1982, 217).
What is rather astonishing is the ban on new uses of known products. Under the European system this is not excluded a priori as is apparently the case in the above Indian law, but rather ,may be denied a posteriori if the new use of the known product is intimately related to the known one, such that it no longer complies with the inventive step requirement. However, where known and new uses are sufficiently different no inventive step objection arises and the new use is patentable. For example nitroglycerine was known as an explosive - consequently its use as a detonator for other explosives is intimately related to its known use and as such would lack inventive step, however, it was also later discovered that nitro-glycerine had therapeutic effects of the cardiovascular system and this new totally unrelated use would have been inventive according to European law.
Efficacy is beside the point. Novartis itself says on its corporate citizenship website that "Our case in India is solely about safeguarding intellectual property."
ReplyDeleteThe question nobody has answered is whether granting Novartis a patent on the reformulation of Glivec would preclude local manufacturers from producing generic versions of the previous formulation.
Your post assumes that it wouldn't, but I can't imagine Novartis would bring the suit if winning wouldn't have that effect.
A patent on a reformulated Glivec could not be used to prevent previously known formulations (i.e. known before the earliest priority date of the patent) from being made and sold. This is fundamental to the concept of patents. One cannot be granted a valid patent for something that was publically known before.
ReplyDeleteOnce an Intermediate, always an Intermediate?
ReplyDeleteThe other day, while I was having a heated discussion with 2 fellow bloggers about the patentability of repurposed drugs i.e. patenting of new use of a known drug, we hit a road block with regard to patent value of an intermediate. The question raised was "Will a newly found first use of an already known intermediate be patentable in India?"
I am rephrasing the question for the purpose of this discussion.
“Will the exclusion criterion elaborated in Section 3 (d) of the Indian Patents Act exclude the patentability of the first known use of the intermediate?”
Whenever the exclusion pertaining to new use of... to read on log on to www.sinapseblog.com