 Yesterday was a good day for Patents Court (England and Wales) judge Mr Justice Arnold. First, in Mylan v Yeda (noted here by the IPKat), his decision was upheld by a Court of Appeal containing two specialist IP judges. Then, no doubt by sheer coincidence, another version of the same Court of Appeal, this time consisting of Lords Justices Longmore, Moore Bick and Floyd, went and upheld him again in Resolution Chemicals Ltd v H. Lundbeck A/S [2013] EWCA Civ 924.
Yesterday was a good day for Patents Court (England and Wales) judge Mr Justice Arnold. First, in Mylan v Yeda (noted here by the IPKat), his decision was upheld by a Court of Appeal containing two specialist IP judges. Then, no doubt by sheer coincidence, another version of the same Court of Appeal, this time consisting of Lords Justices Longmore, Moore Bick and Floyd, went and upheld him again in Resolution Chemicals Ltd v H. Lundbeck A/S [2013] EWCA Civ 924.
 |
| HMS Resolution: not sunk when earlier Arrow missed its mark |
The Court of Appeal dismissed Lundbeck's appeal. In its view:
* Privity of interest provided an exception to the general principle that estoppel would bind only the parties to previous litigation. The test was whether, having due regard to the subject matter of the dispute, there was a sufficient degree of identity between a party to litigation and a third party so as to act as a bar to the third party commencing a fresh action.
* It was thus for the court to assess on the facts whether there was privity of interest between a new party -- in this case Resolution -- and a party to previous proceedings. In doing so, it had to examine (i) the extent to which the new party had an interest in the subject matter of the previous action; (ii) the extent to which the new party could be said to be, in reality, the party to the original proceedings by reason of its relationship with that party and (iii) against that background, whether it was just that the new party should be bound by the outcome of the previous litigation.
* Arrow and Resolution were indeed part of a group of companies under common control. There was however no subsisting relationship between them under which it could be said that the 2005 proceedings were being conducted by Arrow for Resolution's benefit.
 |
| The IPKat's own earlier experiments didn't get far |
The IPKat thinks Lundbeck can't be blamed for trying its luck here, but it would be difficult to argue with the Court of Appeal's statement of the general principles. Merpel just wonders how many times escitalopram has been litigated across Europe and beyond. If ever a patent was a licence to litigate, this is it!
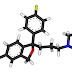 Reviewed by Jeremy
on
Tuesday, July 30, 2013
Rating:
Reviewed by Jeremy
on
Tuesday, July 30, 2013
Rating:



![[Guest post] Can AI be considered a PHOSITA? Policy debates in the US and the EU](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEggxDO6mW5r4n3-06Af5ckmIaMIhzgPJBoDP8AUsSYXY2zajUQt1ObGVn_GhCgidbG_YDVnybQuJ5XoAjjBG9Ws2xJWDQHNPMrLkn526SWIG371X_Kjt1E8tJmn8Ae_20Phyphenhyphen09JeuUOhhTR3aZE9lMRQlqHGQGcAWGxlo91rrKcLk0AfUjWCvw6RQ/s72-c/Two-cats-eating.jpg)












Please expand on the final statement:
ReplyDelete"If ever a patent was a licence to litigate, this is it!"
Anonymous of 12:22. Delighted to oblige: escitalopram has been litigated in France, Germany, Israel, the Netherlands, Italy, Austria, Belgium and Spain, on SPCs alone: see http://thespcblog.blogspot.co.uk/search?q=escitalopram
ReplyDelete...you can add Denmark, Finland, Portugal, Canada and Australia to that list as well.
ReplyDelete