 The concept of novelty in patent law came under scrutiny once again Actavis UK Ltd v Janssen Pharmaceutical NV [2008] EWHC 1422 (Pat), a Patents Court for England and Wales decision yesterday of Mr Justice Floyd.
The concept of novelty in patent law came under scrutiny once again Actavis UK Ltd v Janssen Pharmaceutical NV [2008] EWHC 1422 (Pat), a Patents Court for England and Wales decision yesterday of Mr Justice Floyd.Actavis sought to revoke Merck's patent for an invention involving the sterochemistry of nebivolol, a blood pressure drug, while Merck applied to amend some of the patent's claims. According to Actavis the patent would remain invalid even if the claims were amended, since it was neither new nor inventive. Most of the judgment deals with technical issues rather than broad legal principles so, for the benefit of readers who glaze over when they see all the technical stuff, he's just going to pick out one of the legal issues.
Floyd J ruled that all but one of the disputed claims were invalid and that the application to amend failed. In doing so, he reminded Merck that merely explaining the mechanism which underlay a use that had already been described in the prior art could not, by itself, give rise to novelty, and that not every discovery concerning the mode of action of a drug could be translated into a new purpose and claimed as such. The judge had this to say about claim construction by analogy:
"55 Mr Alexander [for Merck] submitted that the pharmaceutical composition claims (before amendment) were limited to a compound of formula (I) and a single blood pressure reducing agent. He sought to draw an analogy with the construction of the claim to an enantiomer (and a pharmaceutical composition containing it) adopted by Kitchin J in Generics v Lundbeck [2007] EWHC 1040 (Pat), [see here for a note by the IPKat on the Court of Appeal's response to Kitchin J's decision]. Seeking to draw analogies with decided cases about different claims in different patents is not a fruitful or legitimate use of authority. It is sufficient to say that in Generics v Lundbeck the whole point of the invention was the isolation of the enantiomer from its counterpart. In the present case the object of the invention is quite different: using the enantiomer to potentiate other agents, which expressly include the counterpart enantiomer. ..."?
The analogy apart, Mr Alexander submitted that the Court should not, if it can help it, arrive at a construction of the claim which has the result that the claim will read on to prior art referred to in the patent. That is a valuable canon of construction which has been relied on in a number of cases ... but it is not a rigid rule ...".
 Says the IPKat, the judge also makes some telling points about the limited scope of the ruling of the Enlarged Board of Appeal of the European Patent Office in G2/88 MOBIL/Friction reducing additive [1990] EPOR 73, where it was held that
Says the IPKat, the judge also makes some telling points about the limited scope of the ruling of the Enlarged Board of Appeal of the European Patent Office in G2/88 MOBIL/Friction reducing additive [1990] EPOR 73, where it was held that "In relation to a claim whose wording clearly defines a new use of a known compound, depending upon its particular wording in the context of the remainder of the patent, the proper interpretation of the claim will normally be such that the attaining of a new technical effect which underlies the new use is a technical feature of the claimed invention".He later says (at para 92) "I think there are signs that the courts in the EPO and this country are taking quite a limited view of what Mobil decided", reviewing recent case law and concluding (at para 99) "In my judgement, merely explaining the mechanism which underlies a use already described in the prior art cannot, without more, give rise to novelty. In Mobil, the technical effects which underlay the new and old uses were different and distinct". The original ruling in Mobil was the closest thing to the opening of Pandora's Box that the IPKat can recall, and he suspects that it may be a little while before people stop citing it as authority for a variety of propositions that it has never sought to justify.
 Reviewed by Jeremy
on
Tuesday, July 01, 2008
Rating:
Reviewed by Jeremy
on
Tuesday, July 01, 2008
Rating:




![[GuestPost] G1/24: Tuning in! A take on the state of proceedings before oral proceedings](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjZhEivE5bp7QOwZsyZXAXbVNYSmLjUthkB2Q7fm1_dpB97u5lIQeyWT9ZadUTAH3Z-hXn13VpW4vBDRPx9emCnoDV6tbUTkyvfmqPv1nNInL8XMdrAtSZ2hcRQr2LjxKovC9wTk_XyZxQ0CtX1MUrO_Muz3OJ4ld8AftymsdUmKD7xNksYMwk6/s150/Picture%201.png)

![[Guest book review] The Handbook of Fashion Law (with a discount code)](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgB4h2AdqJKwq9O3Ft4Mb7C39tv_NeFpkzrOfvhIsuwAkM_ops2Hgj7fdwzq_TQsjQDvQrQa-yyC9Q9pNiugseXRlUaMdsr_cmYUbh9lH8HDECMCbsTuNboVgpafyEhkgDkVS6ruHkuz8Sx0QVGI_1S8R9kbsHdNIYrRjqhyphenhyphen010_txjJUYvlZOtWA/s150/Fashion%20Law%20Book%20Bicture.jpg)









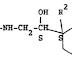
Paragraph 16 needs a bit of a re-write. Someone isn't aware of Fisher projection. Going on to paragraph 17, it is possible to infer what they were trying to say: but they didn't.
ReplyDelete"16 A simple example is tartaric acid which has two chiral centres, and would therefore be expected to have four stereoisomers. In fact it has only three. In the diagram below enantiomers C and D are identical, because rotating one about the vertical axis makes it so: ..."
Bollocks. In the diagram below A and B are identical, because they have a mirror plane, are not chiral and are called meso compounds.
"17 Comp[o]unds which have chiral centres but which are in fact achiral are called meso compounds."
Does the error lie with M'Lud or with Bailii?
Usually, I'm quite impressed by the grasp of science shown (eg Synthon v GSK) by judges.