 A little bird has whispered in the IPKat's ear that there have been further developments in the saga relating to a certain patent for a 70mg weekly dose of alendronate (earlier IPKat posts on the alendronate saga can be found here and here). The Kat now learns that
A little bird has whispered in the IPKat's ear that there have been further developments in the saga relating to a certain patent for a 70mg weekly dose of alendronate (earlier IPKat posts on the alendronate saga can be found here and here). The Kat now learns that "European patent EP1175904B, Merck's patent relating to 70mg weekly alendronate, was declared as lacking inventive step over the prior art by the Opposition Division of the EPO at a hearing held on 17-18 March 2009. It was one of the largest oppositions to be held at the EPO after Merck had used a divisional application to recycle their initial patent on the weekly dosage (EP998292B) after it was revoked by the EPO Technical Board of Appeal in 2006. This patent has generated huge interest within the pharmaceutical industry and had already been the subject of litigation in several EU territories."So now you know.
More on alendronate here
Merck's alendronate patent: the EPO rules
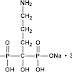 Reviewed by Jeremy
on
Sunday, March 22, 2009
Rating:
Reviewed by Jeremy
on
Sunday, March 22, 2009
Rating:
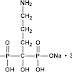 Reviewed by Jeremy
on
Sunday, March 22, 2009
Rating:
Reviewed by Jeremy
on
Sunday, March 22, 2009
Rating:





![[GuestPost] G1/24: Tuning in! A take on the state of proceedings before oral proceedings](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjZhEivE5bp7QOwZsyZXAXbVNYSmLjUthkB2Q7fm1_dpB97u5lIQeyWT9ZadUTAH3Z-hXn13VpW4vBDRPx9emCnoDV6tbUTkyvfmqPv1nNInL8XMdrAtSZ2hcRQr2LjxKovC9wTk_XyZxQ0CtX1MUrO_Muz3OJ4ld8AftymsdUmKD7xNksYMwk6/s150/Picture%201.png)

![[Guest post] ‘Ghiblification’ and the Moral Wrongs of U.S. Copyright Law](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhxl1BQBAW3Y-asjb1xXB9eA4DYy77fky6WgR-prC-_6DeBbDqOgCUDWyiz0Q3B23MWWAXnkbS2H2js7OUwA0JQXAHmsyVFgGIHeJz7zJ791vTzOD-4SJqWFIuywFXQyd3ahybbdZd4e8IEVfcNqctvyR8lumv_Gix6Tsw5cSvbHpTI1nwvztDuAQ/s150/IMG_2179.HEIC)


![[Guest book review] The Handbook of Fashion Law (with a discount code)](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgB4h2AdqJKwq9O3Ft4Mb7C39tv_NeFpkzrOfvhIsuwAkM_ops2Hgj7fdwzq_TQsjQDvQrQa-yyC9Q9pNiugseXRlUaMdsr_cmYUbh9lH8HDECMCbsTuNboVgpafyEhkgDkVS6ruHkuz8Sx0QVGI_1S8R9kbsHdNIYrRjqhyphenhyphen010_txjJUYvlZOtWA/s150/Fashion%20Law%20Book%20Bicture.jpg)






No comments:
All comments must be moderated by a member of the IPKat team before they appear on the blog. Comments will not be allowed if the contravene the IPKat policy that readers' comments should not be obscene or defamatory; they should not consist of ad hominem attacks on members of the blog team or other comment-posters and they should make a constructive contribution to the discussion of the post on which they purport to comment.
It is also the IPKat policy that comments should not be made completely anonymously, and users should use a consistent name or pseudonym (which should not itself be defamatory or obscene, or that of another real person), either in the "identity" field, or at the beginning of the comment. Current practice is to, however, allow a limited number of comments that contravene this policy, provided that the comment has a high degree of relevance and the comment chain does not become too difficult to follow.
Learn more here: http://ipkitten.blogspot.com/p/want-to-complain.html