While this guest Kat was enjoying the Easter Monday public holiday in the UK, the US Courts were busy issuing decisions, and so today the IPKat's attention was drawn by an
article on Bloomberg to this
decision issued on 9 April 2012 by the Court of Appeals for the Federal Circuit.
The decision relates to a dispute between Sanofi-Aventis (Plaintiff) and Hospira and Apotex (Defendants) concerning two patents US 5750561 and US 5714512 and relating to formulations of the anti-cancer drug docetaxel, sold under the trade mark Taxotere. Docetaxel is covered by expired patent US 4814470. This particular Kat has a particular affection for docetaxel and the similar drug paclitaxel (Taxol) because they were of interest to colleagues of his when he was performing his DPhil research. Taxol is well known in UK patent circles for its part in two cases
Bristol-Myers Squibb Company v Baker Norton Pharmaceuticals Inc and Napro Biotherapeutics Inc, which was about an administration regime, and Conor Medsystems Incorporated v Angiotech Pharmaceuticals Incorporated concerning a Taxol-coated stent (which went all the way to the House of Lords).
The two patents at issue here concerned formulations of taxanes (the class of compounds including docetaxel and paclitaxel). Their administration (which is by intravenous infusion) is difficult because they are poorly soluble in water. Additives are used to increase the solubility, for example ethanol, or surfactants such as Cremophor. However, Cremophor was known to sometimes trigger serious allergic reactions, including anaphylactic shock. Both the patents were about reducing use of ethanol and Cremophor.
The claims at issue were Claim 5 of US 5750561:
A perfusion, which contains approximately 1 mg/ml or less of compound of formula as defined in claim 1, and which contains less than 35 ml/l of ethanol and less than 35 ml/l of polysorbate, wherein said perfusion is capable of being injected without anaphylactic or alcohol intoxication mani-festations being associated therewith.
and Claim 7 of US 5714512, whose relevant claims were:
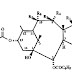
1. A composition comprising a compound of the formula
in which Ar is unsubstituted phenyl, R7 is phenyl or tert butoxy, R6 is hydrogen, R5 is acetyloxy or hydroxy, R3 and R4 taken together form an oxo radical, R1 is hydroxy and R2 is hydrogen, said composition being dissolved in a surfactant selected from polysorbate, polyoxyethylated vegetable oil, and polyethoxylated castor oil, said composition being essentially free or free of ethanol.
6. The composition of claim 1, wherein R5 is hydroxy and R7 is tert butoxy.
7. The composition of claim 6, wherein said surfactant is polysorbate.
The district court found the relevant claims invalid for obviousness (as well as Claim 7 of US 5714512 not infringed). Moreover, both patents were held unenforceable for inequitable conduct.
The appeal decision is in most respects rather routine, as the district court decision is fully upheld.
Noteworthy is perhaps the inequitable conduct finding. It rested, as is so often the case, upon the non-disclosure to the USPTO of two documents that were found to be material to patentability, namely "Vidal" and "CV".
While this is not an Amerikat post, this Kat understands from the present decision that the CAFC last year in the Therasense decision
rejected the “sliding scale” approach to proving inequitable conduct “where a weak showing of intent may be found sufficient based on a strong showing of materiality, and vice versa.” Instead, we instructed that “[i]ntent and materiality are separate requirements.” Additionally, we held that but-for materiality is the standard for evaluating the materiality prong of the analysis unless there is affirmative egregious misconduct.
So, inequitable conduct post-Therasense requires a separate showing of intent and the "but-for" materiality of the non-disclosed documents.
What is but-for materiality?
A prior art reference “is but-for material if the PTO would not have allowed a claim had it been aware of the undisclosed prior art.”
The CAFC found the two documents but-for material, as it had used the references to find the two patents invalid, and therefore considered they were necessarily material.
What is meant by intent?
To satisfy the intent requirement, “the accused infringer must prove by clear and convincing evidence that the applicant knew of the reference, knew that it was material, and made a deliberate decision to withhold it.” In Therasense, we confirmed that inequitable conduct requires clear and convincing evidence of a specific intent to deceive the PTO and that “the specific intent to deceive must be ‘the single most reasonable inference able to be drawn from the evidence.’”
The CAFC affirmed the district court's finding of intent, although it should be remembered that this is not an independent finding of intent, since
In reviewing the district court’s inequitable conduct determination, we review the court’s underlying factual findings for clear error and its ultimate decision as to inequitable conduct for an abuse of discretion.
and
“This court reviews the district court’s factual findings regarding what reasonable inferences may be drawn from the evidence for clear error.”
Reviewing the district court decision, the CAFC said:
In this case, the district court heard extensive testimony from inventor Fabre regarding both the Vidal and GV references, and the court’s finding that Fabre acted with a specific intent to deceive the PTO in withholding those references is not clearly erroneous.
Therefore, the CAFC affirmed the finding of inequitable conduct, stating:
In light of the evidence before the district court supporting the finding of a specific intent to deceive, coupled with the deference we must afford to the district court’s credibility determinations, we cannot conclude that the court’s finding that Fabre withheld the Vidal reference with the specific intent to deceive the PTO was clearly erroneous.
Even though the district court reached its decision before the CAFC decision in Therasense, the CAFC found that the district court had separately determined materiality and intent, and had found both to be present, and the CAFC therefore considered the district court's decision satisfied the Therasense requirements.
This Kat is always saddened by cases involving inequitable conduct, as he suspects that the reality is usually very different from the way things end up appearing years later when the matter is under litigation. At least in this case the patents were found to be invalid on usual patentability grounds anyway.
 Reviewed by Darren Smyth
on
Tuesday, April 10, 2012
Rating:
Reviewed by Darren Smyth
on
Tuesday, April 10, 2012
Rating:






![[GuestPost] G1/24: Tuning in! A take on the state of proceedings before oral proceedings](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjZhEivE5bp7QOwZsyZXAXbVNYSmLjUthkB2Q7fm1_dpB97u5lIQeyWT9ZadUTAH3Z-hXn13VpW4vBDRPx9emCnoDV6tbUTkyvfmqPv1nNInL8XMdrAtSZ2hcRQr2LjxKovC9wTk_XyZxQ0CtX1MUrO_Muz3OJ4ld8AftymsdUmKD7xNksYMwk6/s150/Picture%201.png)
![[Guest post] ‘Ghiblification’ and the Moral Wrongs of U.S. Copyright Law](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhxl1BQBAW3Y-asjb1xXB9eA4DYy77fky6WgR-prC-_6DeBbDqOgCUDWyiz0Q3B23MWWAXnkbS2H2js7OUwA0JQXAHmsyVFgGIHeJz7zJ791vTzOD-4SJqWFIuywFXQyd3ahybbdZd4e8IEVfcNqctvyR8lumv_Gix6Tsw5cSvbHpTI1nwvztDuAQ/s150/IMG_2179.HEIC)



![[Guest book review] The Handbook of Fashion Law (with a discount code)](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgB4h2AdqJKwq9O3Ft4Mb7C39tv_NeFpkzrOfvhIsuwAkM_ops2Hgj7fdwzq_TQsjQDvQrQa-yyC9Q9pNiugseXRlUaMdsr_cmYUbh9lH8HDECMCbsTuNboVgpafyEhkgDkVS6ruHkuz8Sx0QVGI_1S8R9kbsHdNIYrRjqhyphenhyphen010_txjJUYvlZOtWA/s150/Fashion%20Law%20Book%20Bicture.jpg)






Is but-for materiality also known as 20/20 hindsight?
ReplyDelete